
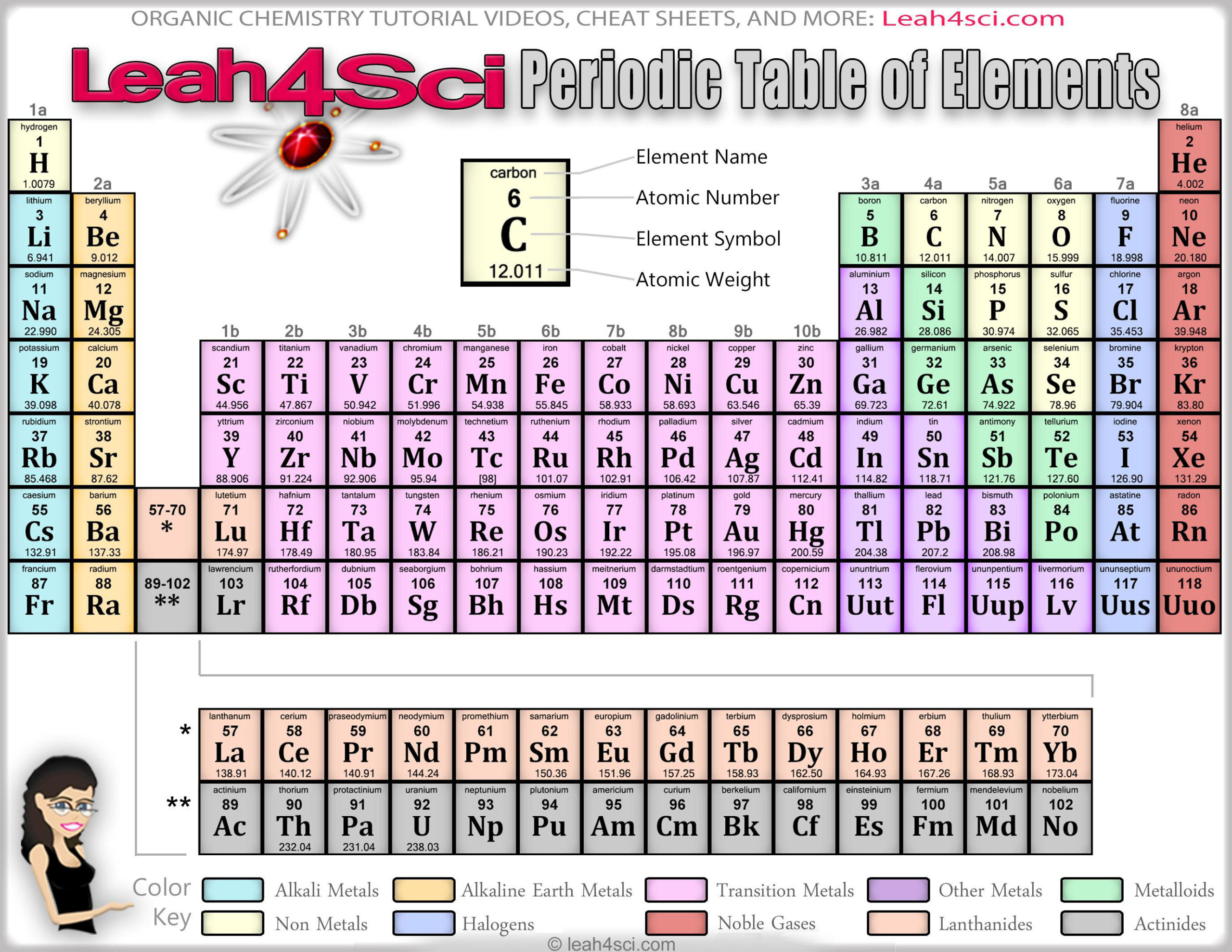
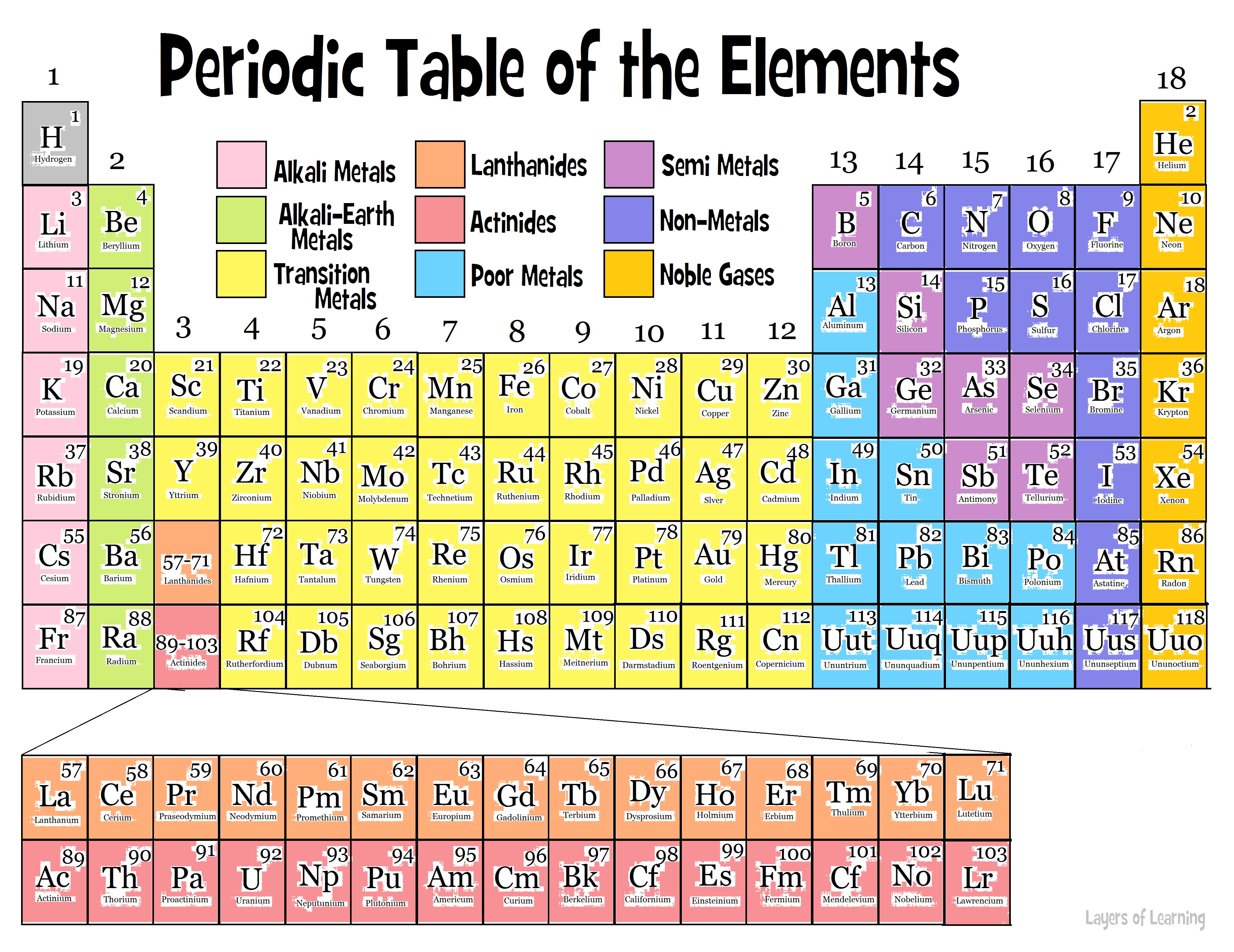
They all have a similar electron configuration in their valence shells: a single s electron. Their electron configurations (abbreviated for the larger atoms) are as follows, with the valence shell electron configuration highlighted: Table 8.4 Electron Configurations of Elements in the First Column of the Periodic Table Element For example, take the elements in the first column of the periodic table: H, Li, Na, K, Rb, and Cs. If we look at just the valence shell’s electron configuration, we find that in each column, the valence shell’s electron configuration is the same. (The inner electrons are called core electrons.) The valence electrons largely control the chemistry of an atom.
P PERIODIC TABLE PLUS
The electrons in the highest-numbered shell, plus any electrons in the last unfilled subshell, are called valence electrons the highest-numbered shell is called the valence shell. Instead of filling the 3 d subshell next, electrons go into the 4 s subshell, which consists of K and Ca (Figure 8.18 “The 4 s Subshell”).įigure 8.20 “Blocks on the Periodic Table.” The periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. Figure 8.17 “The 3 p Subshell.” Next, the 3 p subshell is filled with electrons. Next, the 3 p subshell is filled with the next six elements (Figure 8.17 “The 3 p Subshell”). Figure 8.16 “The 3 s Subshell.” Now the 3 s subshell is being occupied. The elements when this subshell is being filled, Na and Mg, are back on the left side of the periodic table (Figure 8.16 “The 3 s Subshell”). The next subshell to be filled is the 3 s subshell. Figure 8.15 “The 2 p Subshell.” For B through Ne, the 2 p subshell is being occupied. On the right side of the periodic table, these six elements (B through Ne) are grouped together (Figure 8.15 “The 2 p Subshell”). Figure 8.14 “The 2 s Subshell.” In Li and Be, the 2 s subshell is being filled.įor the next six elements, the 2 p subshell is being occupied with electrons. Figure 8.14 “The 2 s Subshell” shows that these two elements are adjacent on the periodic table. The next two electrons, for Li and Be, would go into the 2 s subshell. Figure 8.13 “The 1 s Subshell.” H and He represent the filling of the 1 s subshell. These two elements make up the first row of the periodic table (see Figure 8.13 “The 1 s Subshell”). Their electron configurations are 1 s 1 and 1 s 2, respectively with He, the n = 1 shell is filled. Why does the periodic table have the structure it does? The answer is rather simple, if you understand electron configurations: the shape of the periodic table mimics the filling of the subshells with electrons.

Figure 8.12 “The Periodic Table.” View an accessible periodic table online. A periodic table is shown in Figure 8.12 “The Periodic Table.” The elements are listed by atomic number (the number of protons in the nucleus), and elements with similar chemical properties are grouped together in columns. In Chapter 3 “Atoms, Molecules, and Ions”, we introduced the periodic table as a tool for organizing the known chemical elements.
The honour is also in recognition for him acting as an ambassador for UK Science in his role as Vice-President and Foreign Secretary of the Royal Society, which is an illustration of the esteem in which he is held by his fellow academics. He received the honour for services to Chemical Sciences in recognition of his contribution as a global leader in green and sustainable chemistry.
He is a renowned professor at The University of Nottingham, and was knighted in the Queen’s New Year Honours 2015. Sir Martyn was a special guest at the launch ceremony of the International Year of the Periodic Table at UNESCO in Paris. The hugely popular videos have made Sir Martyn and his team YouTube stars with millions of fans worldwide.
P PERIODIC TABLE UPDATE
Since the first video was posted the team has filmed experiments for most of the elements and regularly update the videos with new stories, better samples and bigger experiments. Over the years the Periodic Table has been used in many creative and fun ways to engage audiences with science and in 2008 Sir Martyn Poliakoff teamed up with video journalist Brady Haran to start the Periodic Table of Videos.


 0 kommentar(er)
0 kommentar(er)
